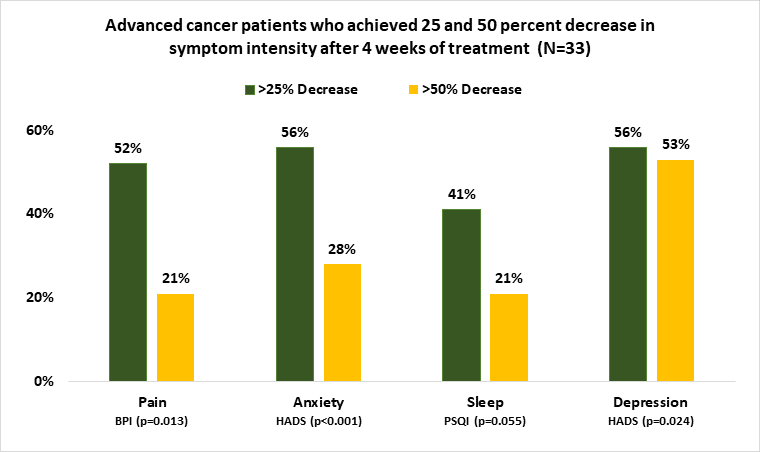
The latest clinical research study proving the effectiveness of Alpha-Stim focuses on a segment of the population that is routinely prescribed medications for pain, anxiety, insomnia and depression—cancer patients.
The study was conducted by the MD Anderson Cancer Center, one of the largest and most prominent cancer research centers in the world and pre-released by the Journal of Pain and Symptom Management. It found that advanced cancer patients who were treated with cranial electrotherapy stimulation (CES) via a handheld, non-invasive medical device called Alpha-Stim for just four weeks, saw significant improvement of pain, anxiety, depression and sleep.
While Alpha-Stim is FDA cleared as a fast, safe and proven effective treatment, and has more clinical research than any other device in its class, there are no published studies in patients with advanced cancer. The study examined the safety and efficacy of this non-drug treatment option, affirming years of research that Alpha-Stim is extremely effective, with no serious adverse effects and no risk of addiction, unlike opioids.
Daniel L. Kirsch, PhD, inventor of the Alpha-Stim and Chairman of Electromedical Products International, Inc., noted that one of the most powerful things about the current study is that it asserts the power of Alpha-Stim to go beyond typical prescription medications.
“I am very happy to see that Alpha-Stim technology has been able to help advanced cancer patients on multiple issues during the most difficult period in their lives, with a study that proves it,” Kirsch said. “Since it was first introduced in 1981, many psychiatrists, most peer reviewers, insurance adjusters and all government bureaucrats have said that it might be effective in some cases of mild depression but for real disorders you need drugs. If they admitted it worked at all.”
Kirsch, a board-certified pain specialist, said that while the general belief has been that drugs are the most effective treatment, this study serves as a reminder that—even for advanced cancer patients who are routinely given prescription medications—the regular drugs that are being prescribed do not provide adequate treatment.
“The data gleaned from 36 years of use, over 15 million people treated, and from over 100 research studies tells a different truth,” Kirsch said. “In fact, we live in a drug culture. Nearly everyone who uses Alpha-Stim was already on drugs, but they either did not get an adequate effect from drugs or couldn’t tolerate their side effects. If drugs were safe and effective, there would be no Alpha-Stim. As clearly stated in this study, the effect was over and above that of the drugs they were on.”
Jeffrey Marksberry, MD, Vice President of Science and Education at EPI, noted that this study is especially important as Americans struggle with an on-going opioid epidemic that is fueled by current prescription medications.
“We are very proud and excited about this study from MD Anderson,” Marksberry said. “There is a current paradigm shift in medicine, moving away from medications riddled with side effects and addictive properties as is evident by the daily mention of the opioid epidemic on the nightly news. In order to reduce our national dependence on prescription medication, there have to be viable options available for practitioners and their suffering patients. The MD Anderson study proves that Alpha-Stim is a viable treatment option for pain, anxiety, insomnia and depression in one of the most difficult to treat patient populations.”
Click here to read the pre-released version of the study from MD Anderson Cancer Center on “Cranial Electrotherapy Stimulation for the Management of Depression, Anxiety, Sleep Disturbance, and Pain in Patients with Advanced Cancer: A preliminary study,” published by the Journal of Pain and Symptom Management.
Click here to learn more or get started on your journey with Alpha-Stim!