CLINICAL RESEARCH
Safety, Impressive Results, and Long-Term Effectiveness
Physician and patient surveys that show 90% of the people who use Alpha-Stim® get significant relief when using it as a form of pain or insomnia treatment. This is supported by over 95 completed independent research studies and published reports, many of which were randomly controlled trials to ensure rigorous testing and clinically validated results. That’s why over 200 Department of Defense (DOD) practitioners and over 92 Veterans Administration (VA) hospitals use Alpha-Stim with military personnel for acute, chronic, and post-traumatic pain and insomnia treatment.
Reduced Pain
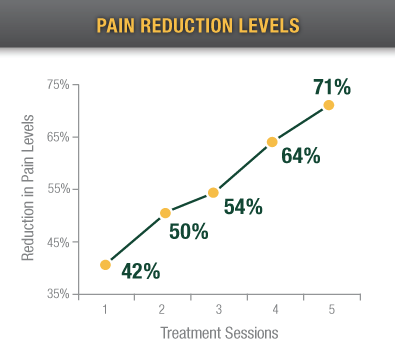
In a study of severe pain patients, Alpha-Stim significantly reduced pain by an average of 71% after only 5 treatments.1
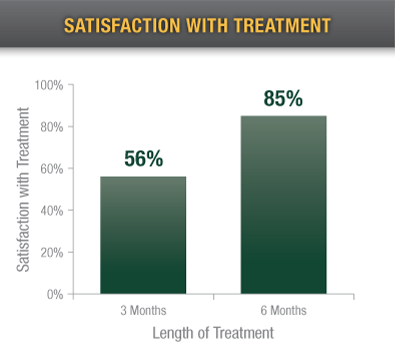
In a post-traumatic pain study, 56% of patients at 3 months and 85% at 6 months were somewhat or very satisfied with the device.2
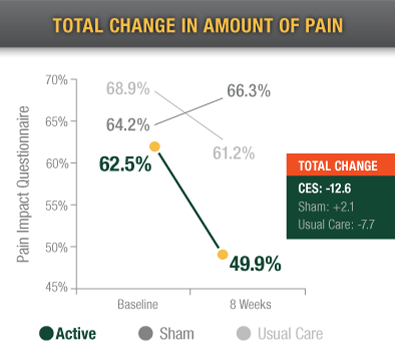
Chronic pain patients using Alpha-Stim reported significantly improved functionality than the usual care and sham groups.3
Reduced Insomnia
Increased Sleep Time: Alpha-Stim CES recipients demonstrated an average increase of 43 total minutes of sleep time after only 5 treatments*4
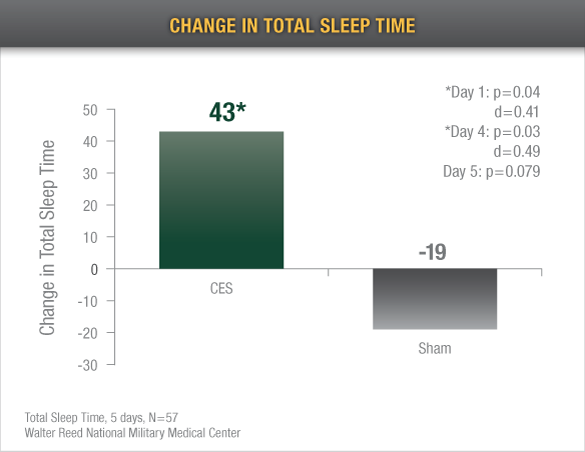
*Insomnia reduction varies widely, with some individuals having improved sleep immediately and others not having improved sleep until weeks into treatment.
Reduced Sleep Disturbances: Alpha-Stim CES reduced sleep disturbances with patients, completing this 8-week study with scores below the range of insomnia5
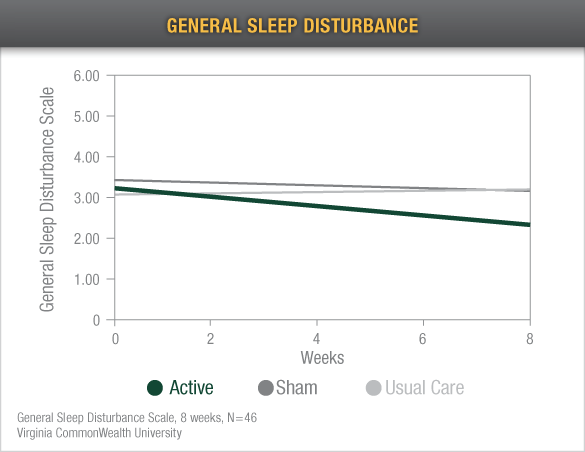
Safety
Alpha-Stim has proven its safety in 95 research studies, published reports and ongoing research, physician and patient surveys. Scores of professionals who have used Alpha-Stim technology for pain or insomnia treatment have remarked on the unsurpassed safety and quality of the devices.
In over 30 years of studies involving 8,800 people, only minor side effects have been reported. The two most common side effects were headaches (0.10%) and skin irritation at the electrode sites (0.07%, only seen in light-skinned people).
Patients who reported a positive response, according to: 2011 WebMD Drug Surveys, 2011 Alpha-Stim Service Member Survey (N=152), and 2011 Alpha-Stim Civilian Patient Survey (N=1,745).6,7
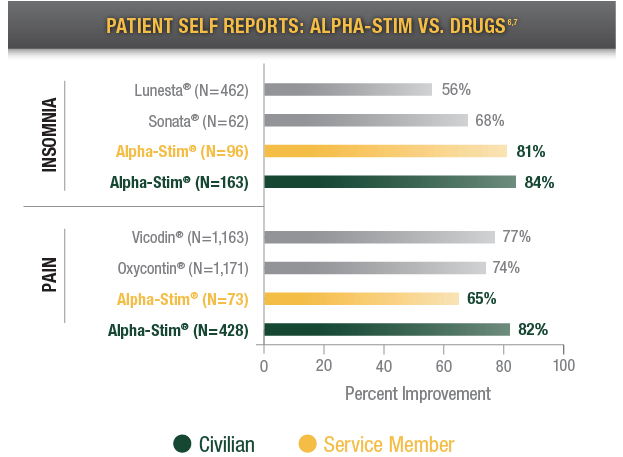
To see a complete Alpha-Stim bibliography with annotated abstracts and published studies, contact customer service at (940) 328-0788 or by emailing alpha-stim@epii.com
- Holubec JT. Cumulative response from Cranial Electrotherapy Stimulation (CES) for chronic pain. Practical Pain Management. 2009; 9(9):80-83.
- Tan G, Rintala D, Jensen MP, et al. Efficacy of Cranial Electrotherapy Stimulation for neuropathic pain following spinal cord injury: a multi-site randomized controlled trial with a secondary 6-month open-label phase. Journal of Spinal Cord Medicine. 2011; 34(3):285-296.
- Taylor AG, Anderson JG, Riedel SL, et al. Cranial Electrotherapy Stimulation improves symptoms and functional status in individuals with fibromyalgia. Pain Management Nursing. 2013 Dec; 14(4):327-335.
- Lande, R. Gregory and Gragnani, Cynthia. Efficacy of cranial electric stimulation for the treatment of insomnia: A randomized pilot study. Complementary Therapies in Medicine. 2013; 21(1):8-13.
- Taylor AG, Anderson JG, Riedel S L., Lewis, et al. Cranial electrical stimulation improves symptoms and functional status in individuals with fibromyalgia. Pain Management Nursing. 2013; Dec;14(4):327-35.
- Accessed online October 28, 2011 at www.WebMD.com/drugs. Pharmaceutical survey data.
- Alpha-Stim Military Service Member and Civilian Data. 2011. Conducted by Larry Price, PhD, Associate Dean of Research and Professor of Psychometrics and Statistics, Texas State University.
