WHEN YOUR PATIENTS NEED RELIEF
Let Nothing Stop Them™
The Alpha-Stim® AID and Alpha-Stim M devices can help your patients reduce pain, feel better, and live better, quickly and safely.
Call or email for practitioner volume discount pricing at 1-800-FOR-PAIN, or info@epii.com
To view additional Alpha-Stim research and clinical indications, please enter your password here:
Licensed healthcare professionals may obtain a password free by contacting customer service at (940) 328-0788 Ext. 3,
or by emailing info@alpha-stim.com
Licensed by Health Canada, the Alpha-Stim Cranial Electrotherapy Stimulation (CES) device and Microcurrent Electrical Therapy (MET) devices has helped hundreds of thousands of people achieve remarkable results. Surveys of Alpha-Stim users show overwhelming evidence of the effectiveness of the Alpha-Stim MET and CES device for treating chronic and acute pain, as well as insomnia, listed below:
% Reporting Significant Improvement (>25%)*
Pain
Insomnia
*Patient self-reports after using Alpha-Stim technology for at least 3 weeks.
Alpha-Stim is supported by over 95 completed independent research studies and published reports that utilize some of the most rigorous, controlled clinical study methods. Again and again, Alpha-Stim has proven to quickly and safely provide relief from pain and insomnia.
Alpha-Stim AID
Quickly and safely help patients get relief from symptoms of insomnia
Alpha-Stim M
Quickly and safely deliver sustainable pain relief along with relief from symptoms of insomnia
Why You Should Prescribe Alpha-Stim
You can confidently prescribe the Alpha-Stim MET and CES devices to supplement medications for treating chronic pain, acute pain, post-traumatic pain, and insomnia. You can also use it as first-line therapy for patients unresponsive—or not sufficiently responsive—to medications.
Used appropriately, Alpha-Stim M and AID may provide significant symptom improvements for many of your patients:
- Patients suffering from pain or insomnia can enjoy noticeable relief after just one treatment
- Once you’ve gotten your patients’ condition(s) under control, you can decrease use of the device. Alpha-Stim is that powerful.
Alpha-Stim Patented Waveform
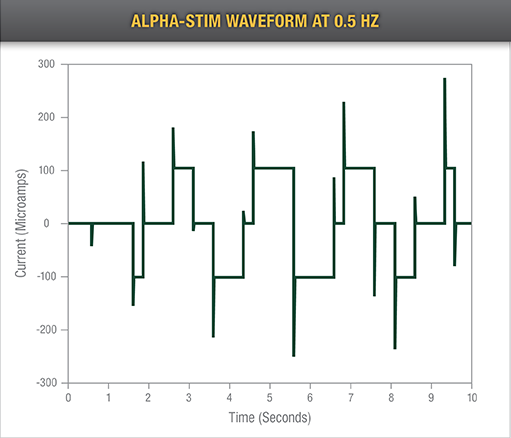
Proven Efficacy
The Alpha-Stim CES device delivers solid relief to patients time after time, treating their chronic and acute pain, as demonstrated in numerous clinical studies conducted over 30+ years.
Alpha-Stim’s ability to deliver beneficial effects after a single treatment abounds in fMRI, LORETA and EEG mechanistic studies and clinical double-blind, randomly-controlled medical, psychological, and dental studies.1-11
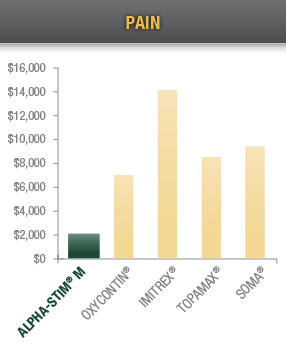
Cost Effectiveness
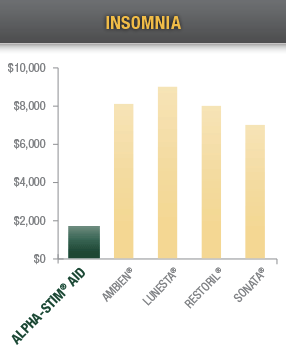
Over a five year period, treating chronic pain, acute pain, and post-traumatic pain with Alpha-Stim CES and MET devices saves your patients thousands of dollars compared with drugs typically prescribed for insomnia and pain.12
To view additional Alpha-Stim research and clinical indications, please enter your password here:
Licensed healthcare professionals may obtain a password free by contacting customer service at (940) 328-0788 Ext. 3, or by emailing info@alpha-stim.com
Although rare, please note that Alpha-Stim may put patients into a euphoric state that temporarily limits their mental and/or physical abilities to perform potentially dangerous tasks for several hours. Advise them to be careful when operating a car, truck, or heavy machinery until they know how Alpha-Stim affects them.
You won’t have to worry about lasting side effects. None have been reported. Some patients get occasional self-limiting reactions such as lightheadedness, headaches, or skin irritation from the electrodes.
References
- Feusner JD, Madsen S, Moody T, et al. Effects of Cranial Electrotherapy Stimulation on resting state brain activity. Brain and Behavior. 2012;1-10.
- Taylor AG, Anderson JG, Riedel SL, et al. A randomized, controlled double-blind pilot study of the effects of Cranial Electrotherapy Stimulation on activity in brain pain processing regions in individuals with fibromyalgia. Explore. 2013; 9(1):32-40.
- Kennerly R. Changes in quantitative EEG and low resolution tomography following Cranial Electrotherapy Stimulation. PhD Dissertation, the University of North Texas. 2006; 529 pp., 81 tables, 233 figures, 171 references.
- Chen Y, Yu L, Zhang J, et al. Results of Cranial Electrotherapy Stimulation to children with mixed anxiety and depressive disorder. Shanghai Archives of Psychiatry. 2007; 19(4):203-05.
- Lande RG, Gragnani C. Efficacy of Cranial Electrotherapy Stimulation for the treatment of insomnia: A randomized pilot study. Complementary Therapies in Medicine. 2013; 21(1):8-13.
- Cork RC, Wood P, Ming N, et al. The effect of Cranial Electrotherapy Stimulation (CES) on pain associated with fibromyalgia. The Internet Journal of Anesthesiology. 2004; 8(2).
- Rintala DH, Tan G, Willson P, et al. Feasibility of using Cranial Electrotherapy Stimulation for pain in persons with parkinson’s disease. Parkinson’s Disease. 2010; 8 pages.
- Barclay TH, Barclay RD. A clinical trial of Cranial Electrotherapy Stimulation for anxiety and comorbid depression. Journal of Affective Disorders. 2014; 164:171-77.
- Mellen RR, Mackey W. Reducing sheriff’s officer’s symptoms of depression using Cranial Electrotherapy Stimulation (CES): A control experimental study. The Correctional Psychologist. 2009; 41(1):9-15.
- Winick RL. Cranial Electrotherapy Stimulation (CES): A safe and effective low cost means of anxiety control in a dental practice. General Dentistry. 1999; 47(1):50-55.
- Kolesos On, Osionwo Ho, Akkhigbe. The role of relaxation therapy and Cranial Electrotherapy Stimulation in the management of dental anxiety in Nigeria. ISOR Journal of Dental and Medical Sciences. 2013; 10(4):51-57.
- www.drugpriceinfo.com
