Tan, G., Rintala, D., Herrington, R., Yang, J., Wade, W., Vasilev, C. and Shanti, B.F. Treating Spinal Cord Injury Pain With Cranial Electrotherapy Stimulation. Journal of Spinal Cord Medicine. 2003; 26(3). Poster presented at the Annual Meeting of the American Paraplegia Society, Las Vegas, Nevada, September 2-4, 2003. Download Poster
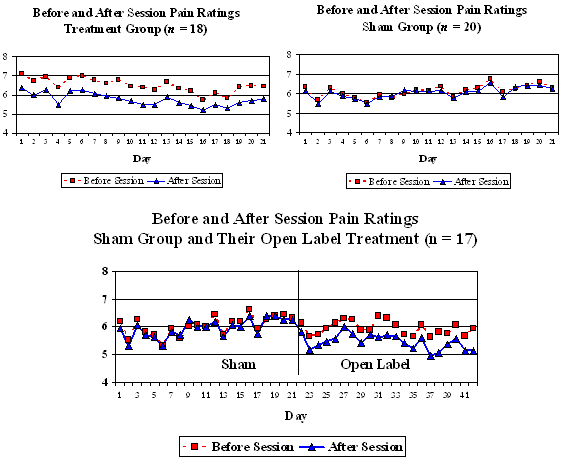
A double–blind, sham-controlled, pilot study was undertaken in which 38 veterans at the VA Medical Center in Houston, Texas with spinal cord injuries (SCI) with neuropathic or musculoskeletal pain were randomly assigned to either a treatment (n = 18) or a control group (n = 20). They were trained to self-administer Alpha-Stim CES treatment in the comfort of their home. The treatment group received one hour per day of 100 microampere sub-sensation CES for a total of three consecutive weeks (21 days). The control group received sham CES for the same amount of time. After completion of the treatment, participants in the control group were offered an open label use of the device for 21 days. They again completed pain ratings before and after each daily session. The average change in pain on a scale from 0 to 10 from before to after each session across the 21 days was .73 for the active CES treatment group and .08 for the sham treatment group (Figures 1 and 2). This was a significant difference (t separate = 2.27, p < .034, effect size = .135). In separate t-tests, there was a significant difference between the before-session and after-session pain ratings for the active treatment group (mean before = 6.46, mean after = 5.73, t = 2.69, p< .016), but the difference between the before and after ratings for the sham treatment group was not significant (mean before = 6.08, mean after = 6.00, t = 0.98, p< .337).
Furthermore, when 17 participants from the group that originally had the sham treatment, participated in the subsequent open-label phase (3 people declined the open-label phase), there was a significant difference between the before and after ratings (Figure 3, mean before = 5.97, mean after = 5.51, t = 3.47, p< .003).
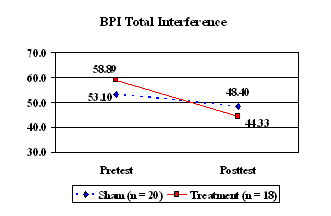
The participants in both groups also completed the Brief Pain Inventory before and after the 21-day treatment phase. The group who received the active CES treatment reported significantly less interference in activities due to pain after completing the intervention compared with prior to the intervention (Figure 4, t = 3.31, p < .004). There was no significant change in pain interference for the group who received sham CES (t = 1.22, p < .238).
Based on both reported pain reductions pre and post each session as well as pre and post the full series of treatments, Alpha-Stim CES treatment was found to be significantly more efficacious than the sham treatment with a moderate to large effect size. The findings also indicate significant reduction in pain interference and catastrophizing after CES treatment.
Figures 1, 2, and 3: Daily Pain Rating for Active CES and Sham CES Groups